VN_bigPicture
CEFAZOLIN Na Sterile
Crystalline sterile powder
For Parenteral Administration

MW: 476.5
-CAS-REGISTRY No: 27164-46-1
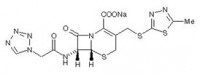
- MOLECULAR FORMULA: C14H13N8NaO4S3
- CHEMIDAL NAME:
Sodium
(6R,7R)-3-[[(5-methyl-1,3,4-thiadiazol-2-yl)thio]methyl]-8-oxo-7-[(1H-tetrazol-1-ylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic
acid.
CATEGORAY:
SPECIFICATION:
- Description:
White to off-white, practically
odorless, crystalline powder, or white to off-white solid
- pH: Between 4.0 and 6.0
- Water content (Karl Fischer Test): N.M.T. 6.0% w/w
- Particulate
Matter (visual): DAC Prob 5 SE<= 4.5
- Particulate Matter (HIAC): ³ 10mm N. M.
T. 6000 Particles, ³ 25mm N. M.
T. 600 Particles
- Sterility:
sterile
- Bacterial endotoxine: N.M.T. 0.15 E.U./ mg
- Related substance:
- Individual Impurity: N. M. T. 1.0%
-Total Impurity N. M. T. 3.5%
- Assay: N. L.
T. 89.1% and N.M.T. 110.1%
PACKAGING: 1*10kg in
special aluminum can with type 1 rubber closure and aluminum seal in each
carton.
STORAGE: Preserve in tight container, store in
cool (below 15ºC) and dry place, protect from light
SHELF LIFE: 24 months
PHARMACOPOEIAL QUALITY: USP, BP
REMARKS: N-N-Dimethyl
Anilline and Benzene are not used or generated during the process.
The Referred
Material Complies With BP, EP & USP.






